What we did is, suck out all the air out of a flask filled with our analyte substance. After closing the valve we measure the pressure in there which is the vapour pressure. Do that at different temperatures and you can see what the phase boundary looks like. First thing that amazed me is that we actually got a nice looking graph.

So what do we do with this? We just need the laws of thermodynamics and the ideal gas law. We rearrange them a little bit according to Mr. Clausius and Mr. Clapeyron and get their equation (I am not showing the proof even though it is kind of cool):

By using the chain rule we can also say:
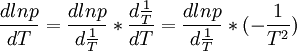
The T2 cancels out and we get:

That means that we get the enthalpy of vaporisation just from the change of vapour pressure with temperature. Who would have thought that? Let's plot the logarithm of the pressure against the reciprocal value of the temperature:

Make a regression, derive, get the enthalpy of vaporisation:

Why is it getting lower? Even that can be explained. At the critical point there is no difference between the phases. Hence there is no enthalpy of vaporisation. You can expect the enthalpy to become smaller when you raise the temperature because you are getting closer to the critical point.
We can even do more. Extrapolate the curve to standard pressure and get the boiling point at 39°C. Now Mr. Trouton tells us that at standard pressure the entropy of vaporisation for every liquid is about 88 J / (mol K). (It seems that this is thermodynamically derived.)
Can we get the entropy just from this curve? Yes we can. We know that ΔvG = ΔvH - TΔvS. Since we are at equilibrium ΔvG = 0. Then ΔvS = ΔvH / T (we actually used this equation already the other way around for deriving the Clausius-Clapeyron equation).
Divide the enthalpy at the boiling point by the temperature and you get 75,8 J / (mol K). That is almost what Mr. Trouton said.
It took me some time to appreciate thermodynamics. But now I think it is amazing how all those values are related and how much you can do with it. Actually synthetic chemistry is cool, too (on paper at least).
2 comments:
I have to admit that synthetic chemistry drives me nuts on occasion, mostly when reactions fail time after time. Thermo might not be the most exciting subject in the world, but some of it is really interesting.
I am not going to start a career in thermodynamics (if that's possible) but it is pretty cool sometimes. Besides that I am always trying to show people how nice things are with a little bit of math. But most of the time they don't believe me ...
Post a Comment