Statistically speaking, writing sequels is not a good idea,[1] but there is something I withheld from you in my previous post about
HOMO-LUMO gaps and excitation energies: the spin of the electron. Spin makes everything a bit more complicated but also more interesting. What are the excitation energies of the singlet and triplet eigenfunctions? And what is exchange splitting?
Compared to last time, we have to construct spin-adapted eigenfunctions. Slater determinants (constructed from restricted orbitals) are always eigenfunctions of S
z but not necessarily of S
2. One simple possibility of creating an S
2 eigenfunction is the construction of a high-spin determinant. And this is how I will start here. For example, we can create a spin-eigenfunction by exciting from spin-down occupied orbital k into the spin-up unoccopied orbital a. As shown in the
previous post, the energy of the resulting determinant is given according to (here the bar marks the spin-down quantities)
The second (exchange type) integral vanishes and the expression can be rewritten as
In other words: In the triplet case the Coulomb interaction between
electron and
hole is the only relevant term.
If we are interested in the singlet then we have to consider linear combinations of excited determinants. The standard construction uses the spin-up and spin-down excited determinants
Here "plus" yields the singlet and "minus" the triplet. The energy of this wavefunction is expanded as:
The first two terms are the energies of the individual Slater determinants (as discussed in the last post). The third term is the coupling element, which is also readily calculated.
The spin-up and spin-down energies are equivalent and of the coupling terms only the first (exchange-like) one remains:
If we use the energy expression of the excited Slater determinant from the
previous post we obtain for the singlet energy
When compared to the simple band picture, the energy is lowered by the Coulomb interaction between the
electron and
hole and raised by twice the exchange interaction. For the triplet we obtain
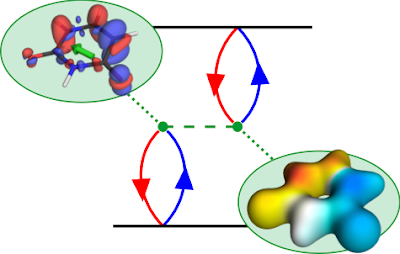
which is (luckily for me trying to write this down) the same as the high-spin triplet discussed above. There is only a Coulomb but no exchange interaction. The splitting between the singlet and triplet amounts to twice the exchange integral, hence it is called "exchange splitting".
The Coulomb term corresponds to the attraction between the
hole density (computed as the square of orbital k) and the
electron density (the square of orbital a). It is a long range interaction decaying with the reciprocal distance of the two orbitals. The exchange term is computed by multiplying orbitals k and a with each other (yielding the transition density) and computing the electrostatic repulsion of this with itself. It is a short range interaction, which requires that a and k occupy the same space. These considerations show that the triplet state will always be lesser or equal in energy than the corresponding
triplet singlet state and that this is particularly pronounced for localized states.
[1] The problem is that there is a very low chance that the sequel to your best post will be your new very best post. Even if it is a good post, it will look bad in comparison to the original one. But this is only a question of the reference point and part of a general phenomenon called
Regression to the Mean.