Merry Christmas everyone! I hope you had a nice celebration (no matter if it was the 24th, 25th or will be the 6th).
There will be a few more dry days on my blog. I'll be gone to Austria's snowless mountains. Snowless thanks to the greenhouse effect [1]. That means no snowboarding for Christmas break [2].
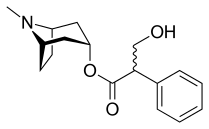
What's coming up next? I am thinking of talking about one or two more molecules. Visualise their structure in PyMOL or POVRay and talk a little bit about them. Then I want to cover some MO-theory. Some more pictures are coming. I will talk about theoretical aspects and proofs, too. Maybe someone's interested. It also helps me to get my thoughts straight if I write about it.
I guess I will see how people are going to like my blog. If I keep getting a few hits per day I'll probably keep writing. Thanks for recently adding my link to a very decent chemist with a cool blog and thanks to some ingenious muppets who aside from adding my link will soon resolve all my problems about fitting together ground-glass joints of different sizes.
[1] It might be just statistical deviation that we had almost no snow so far. But in a materialistic world I needed a catchy phrase to get your attention [3].
[2] I am sorry for everyone who may have been caught in a snow storm in the US or London or anyone else who has had bad weather conditions. That is of course much worse.
[3] In spite of the fact that Austria's snowless mountains may not be caused by the greenhouse effect, I am still worried by it. Here is my suggestion to anyone reading this: Try taking your bike to work for once. Not only is it good for the environment and cheaper but it is also nice for athletic reasons [4].
[4] To be honest: I stole the idea of making footnotes of footnotes from carbon-based curiosities. But the idea of making footnotes of footnotes of footnotes I just came up with myself.
Onnit's Instant Melatonin Spray Keeps Bedtime Uncomplicated
-
Just spritz and sleep—no melatonin tablets or pills necessary.
8 hours ago